幼年二倍体和三倍体北极红点鲑Salvelinus alpinus对穷尽运动的生理反应
The physiological response of juvenile diploid and triploid Arctic charr Salvelinus alpinus to exhaustive exercise
三倍体是生产不育鱼的有效工具,但通常会导致商业水产养殖性能受损。有鉴于此,我们的研究比较了幼年二倍体和三倍体北极红点鲑 Salvelinus alpinus 对穷尽运动的生理反应,北极红点鲑是一种具有巨大水产养殖潜力的极地物种。标准斜坡游泳方案显示临界游泳速度(U暴击) 倍性之间。倍性对U后也没有影响暴击血糖、乳酸或血细胞比容。然而,三倍体的红细胞核分割频率明显更高。与倍性无关,血乳酸水平与U暴击.我们得出的结论是,三倍体不会损害幼年高山沙门虫对穷尽运动的反应。
水产养殖,红细胞,运动,极地,鲑科鱼类,三倍体
Triploidy is an effective tool for producing sterile fishes but often results in impaired performance in commercial aquaculture. In light of this, our study compared the physiological response to exhaustive exercise in juvenile diploid and triploid Arctic charr Salvelinus alpinus, a polar species with great potential for aquaculture. A standard ramping swimming protocol revealed no significant difference in critical swimming velocity (Ucrit) between ploidies. There was also no effect of ploidy on post-Ucrit blood glucose, lactate or haematocrit. However, triploids had a significantly higher frequency of erythrocyte nuclear segmentation. Independent of ploidy, there was also a significant positive correlation between blood lactate levels and Ucrit. We conclude that triploidy does not impair the response to exhaustive exercise in juvenile S. alpinus.
aquaculture, erythrocyte, exercise, polar, salmonid, triploidy
1 引言
北极红点鲑 Salvelinus alpinus L. 是一种冷水鲑鱼,原产于北半球的北极、北方和温带地区,由于其耐受高放养密度、低最佳饲养温度以及高鱼片质量和产量,具有巨大的水产养殖潜力(Sæther 等人,2013 年;Yossa 等人,2019 年)。然而,养殖的高山葡萄容易在收获前发生有性成熟,这一过程将能量分配从体细胞生长转移到性腺,从而降低鱼片产量和质量,并增加疾病易感性(Yossa 等人,2019 年)。此外,正如养殖大西洋鲑鱼 Salmo salar 所记录的那样,在发生养殖逃逸的情况下,它会产生驯化基因组基因渗入当地适应野生种群的风险(Glover 等人,2017 年;Wacker 等人,2023 年;Wringe 等人,2018 年)。
养殖鱼类不必要的性成熟的实用解决方案是使用功能不育的三倍体种群(Benfey,2016 年;Piferrer 等人,2009 年)。然而,与二倍体相比,对三倍体盐酸链球菌的广泛中试和商业试验已经确定了许多福利和农业生产问题,包括白内障、骨骼畸形和总体生存率降低(Fraser 等人,2012 年;Madaro 等人,2022 年;O'Flynn 等人,1997 年)。尽管对三倍体高山链球菌的此类研究较少,但也有类似的证据表明存活率降低(Chiasson 等人,2009 年;Fraser 等人,2022 年)和椎体畸形发生率较高(Fraser 等人,2022 年)。许多研究还表明,三倍体鲑鱼对高温和缺氧等环境压力的耐受能力降低(例如 Benfey 和 Devlin,2018 年;Hansen 等人,2015 年;Jensen 和 Benfey,2022 年;Sambraus 等人,2017 年、2018 年;Scott 等人,2015 年),可能是由于高温下有氧范围减少(Riseth 等人,2020 年)。然而,文献中存在相互矛盾的发现:至少有两项研究未能发现三倍体对高温下有氧范围或最大代谢率的影响(Bowden 等人,2018 年;Sezaki 等人,1991 年)。
红细胞大小的增加通常被认为是三倍体呼吸能力的限制因素,因为与二倍体的较小红细胞相比,这会导致相对于体积的表面积减少(Benfey,1999 年;Benfey 和 Devlin,2018 年)。三倍体鲑鱼也经常表现出较高的红细胞核分割 (ENS) 发生率(Clark 等人,2025 年;Dorafshan 等人,2008 年;Wang 等人,2010 年;Wlasow 等人,2014 年,2004 年),一种来源不明的细胞畸形,定义为细胞核的内侧分裂(Yokote,1982)。 这种畸形尚未在 S. alpinus 中报道。
疫苗接种和分级等常规水产养殖作业要求鱼类在短时间内拥挤,通常与捕获(通过网)和从水中移走相结合。三倍体鲑鱼在初级和次级应激反应方面通常对此反应良好(Benfey & Biron,2000 年;Biron & Benfey,1994 年;Hyndman 等人,2003a;Madaro 等人,2024 年;Preston 等人,2017 年;Sadler 等人,2000 年),尽管如果在高温下被推到筋疲力尽,三倍体可能会表现出高死亡率(Hyndman 等人,2003 年b)。
临界游动速度 (U暴击)是一种简单的测试,经常用于评估鱼类对穷尽运动的生理反应。它与血细胞比容和血血红蛋白水平(Pearson & Stevens,1991)、心输出量(Clark & Seymour,2006)和代谢率(Horodysky 等人,2011 年;Norin &; Clark,2016 年),总体上反映了最大有氧能力(Kolok,1999 年; Norin 和 Clark,2016 年;Plaut,2001 年)。虽然之前的研究表明,U暴击在几种鲑鱼物种中不受三倍体的影响(Bernier 等人,2004 年;Lijalad 和 Powell,2009 年;Riseth 等人,2020 年;Scott 等人,2015 年;Small &; Randall,1989 年;Stillwell &; Benfey, 1997),这尚未在 S. alpinus 中得到检查。
在这项研究中,我们检查了三倍体对这种极地物种对通过 U 进行详尽运动的反应的影响暴击通过运动后采血进行测试。根据之前对其他鲑鱼的研究,我们假设 U暴击二倍体和三倍体之间没有区别。我们试图通过测量继发性应激反应(血糖、乳酸和血细胞比容)来补充这些信息,并调查 S. alpinus 中 ENS 的存在和潜在原因。这些信息可以帮助为这种相对未充分研究的鲑鱼水产养殖物种制定特定物种的养殖场管理实践。
2 材料与方法
2.1 道德声明
这项研究得到了新不伦瑞克大学 (UNB) 动物护理委员会(动物使用协议 24007)的批准,并遵循了加拿大动物护理委员会的所有适用福利和实验指南。
2.2 鱼
二倍体和三倍体 S. alpinus 胚胎是在眼卵阶段从 Valorēs 育种计划(加拿大 Shippagan,NB)购买的。它们是2023年4月人工产卵的3只母(池卵)和10只父(池白子)的后代,在受精后210°C-min开始,通过65.5 MPa的静水压力机处理5 min诱导了大约一半的卵,其余的保留为二倍体对照。三倍体的眼期存活率(发货前)为 58 ± 1%,二倍体为 77 ± 1%。随后,按照标准饲养程序(Jobling 等人,2010 年)在 UNB 水生设施(加拿大新罕布什尔州弗雷德里克顿)饲养鱼类,二倍体和三倍体分别在供应脱氯市政水的流通池中饲养。UNB 的死亡率没有记录,但很低,倍性之间没有明显差异。
2024 年 1 月,在实验前的几个月里,所有鱼都被转移到六缸循环水产养殖系统 (RAS) 内的 314 L 倍性特异性水箱中。该RAS的水质参数保持在推荐范围内(总氨氮和亚硝酸盐均为<1.0 mg/L,硝酸盐为0-400 mg/L,碱度为50-300 mg/L,硬度为>100 mg/L,pH 值为 6.5-8.5;Timmons 等人,2018 年),但单日低碱度 (43 ppm) 除外,通过增加 RAS 的补充水立即得到纠正。在整个 RAS 中,包括直到实验期结束,温度和溶解氧的平均温度和溶解氧平均分别为 10.3°C ± 1.0°C 和 93% ± 5% 的空气饱和度。放养密度约为 109 kg m−3在实验时,个体平均体重约为160克(表1)。用于该实验的所有鱼都来自单个二倍体和单个三倍体水箱。实验前20天,室内季节性调整的光周期从12:12增加到13:11 h(明:暗),以模拟初夏的季节趋势,以维持在单独的RAS内的亲鱼,并且这种13:11状态在整个实验期间保持。
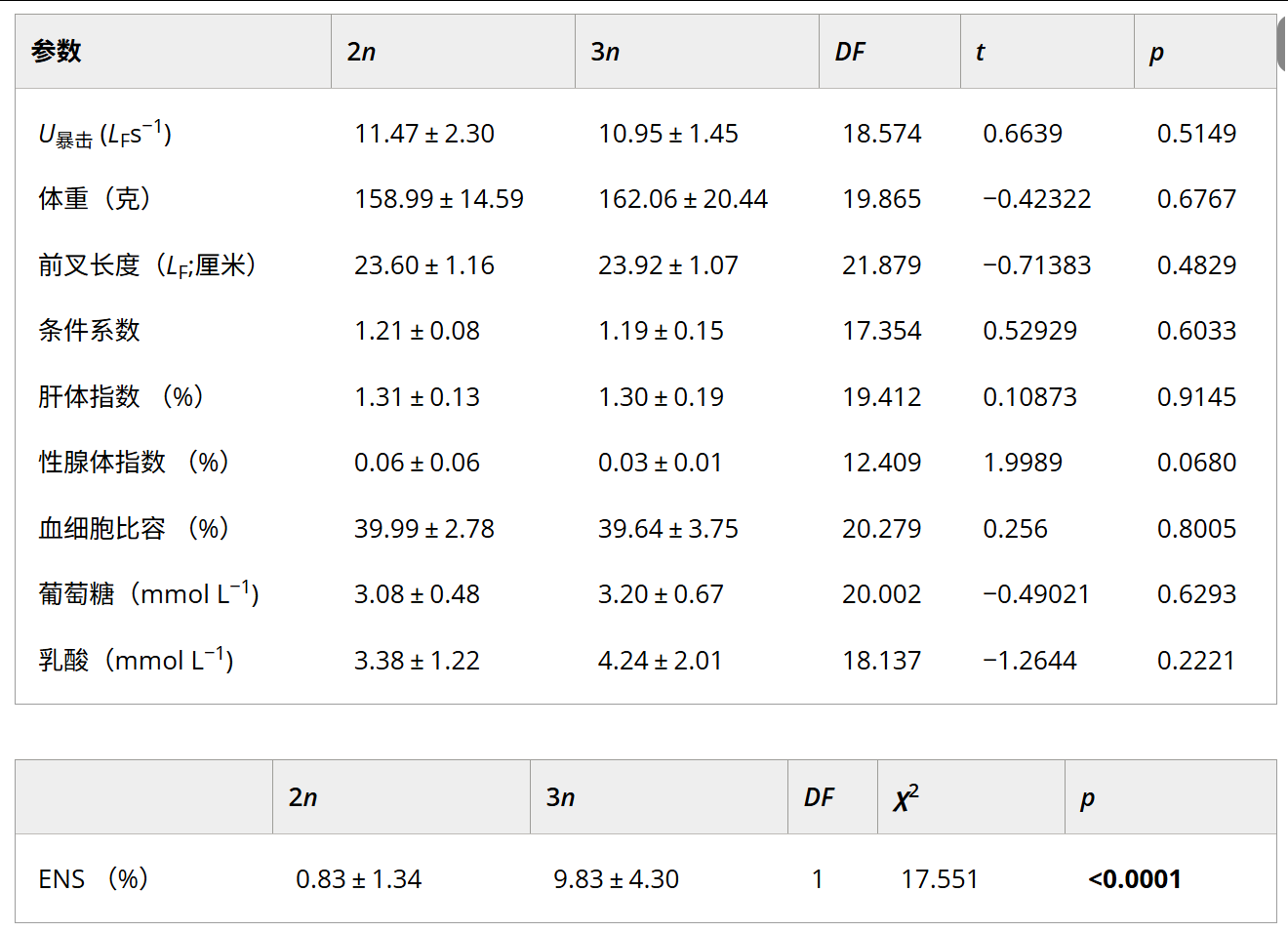
表 1. 临界游动速度 (U暴击)和鱼到达U后立即测量的参数暴击在幼年二倍体 (2n) 和三倍体 (3n) 中 Salvelinus alpinus(平均值 ± 标准差,每个倍性 n = 12)。
1 INTRODUCTION
The Arctic charr Salvelinus alpinus L. is a cold-water salmonid species native to Arctic, boreal and temperate regions of the northern hemisphere with great aquaculture potential due to its tolerance of high stocking densities, low optimum rearing temperature and high fillet quality and yield (Sæther et al., 2013; Yossa et al., 2019). However, farmed S. alpinus are prone to preharvest sexual maturation, a process which diverts energy allocation from somatic growth to the gonads, thereby decreasing fillet yield and quality, and increases disease susceptibility (Yossa et al., 2019). Additionally, it creates the risk of genetic introgression of the domesticated genome into locally adapted wild populations in the event of farm escapes, as documented for farmed Atlantic salmon Salmo salar (Glover et al., 2017; Wacker et al., 2023; Wringe et al., 2018).
A practical solution to unwanted sexual maturation of farmed fishes is to use functionally sterile triploid populations (Benfey, 2016; Piferrer et al., 2009). However, extensive pilot-scale and commercial trials with triploid S. salar have identified numerous welfare and farm production concerns when compared to diploids, including cataracts, skeletal deformities and overall reduced survival (Fraser et al., 2012; Madaro et al., 2022; O'Flynn et al., 1997). Although there have been fewer such studies of triploid S. alpinus, there is similar evidence of reduced survival (Chiasson et al., 2009; Fraser et al., 2022) and higher incidence of vertebral deformities (Fraser et al., 2022). Many studies have also shown triploid salmonids to have a reduced ability to tolerate environmental stressors such as high temperature and hypoxia (e.g. Benfey & Devlin, 2018; Hansen et al., 2015; Jensen & Benfey, 2022; Sambraus et al., 2017, 2018; Scott et al., 2015), possibly due to reduced aerobic scope at elevated temperatures (Riseth et al., 2020). There are, however, conflicting findings in the literature: at least two studies have failed to find such an effect of triploidy on aerobic scope or maximum metabolic rate at elevated temperatures (Bowden et al., 2018; Sezaki et al., 1991).
Increased erythrocyte size is often suggested as the limiting factor to the respiratory capacity of triploids because this results in reduced surface area relative to volume compared to the smaller erythrocytes of diploids (Benfey, 1999; Benfey & Devlin, 2018). Triploid salmonids also frequently exhibit a higher incidence of erythrocyte nuclear segmentation (ENS) (Clark et al., 2025; Dorafshan et al., 2008; Wang et al., 2010; Wlasow et al., 2014, 2004), a cellular deformity of unknown origin that is defined as the medial division of the nucleus (Yokote, 1982). This deformity has yet to be reported in S. alpinus.
Routine aquaculture operations such as vaccination and grading require fish to be crowded for short periods of time, often in combination with capture (by netting) and removal from the water. Triploid salmonids generally respond well to this in terms of their primary and secondary stress response (Benfey & Biron, 2000; Biron & Benfey, 1994; Hyndman et al., 2003a; Madaro et al., 2024; Preston et al., 2017; Sadler et al., 2000) although triploids may exhibit high mortality if pushed to exhaustion at high temperature (Hyndman et al., 2003b).
Critical swimming velocity (Ucrit) is a simple test frequently used to assess physiological responses to exhaustive exercise in fishes. It is positively correlated with haematocrit and blood haemoglobin levels (Pearson & Stevens, 1991), cardiac output (Clark & Seymour, 2006) and metabolic rate (Horodysky et al., 2011; Norin & Clark, 2016), and overall reflects maximum aerobic capacity (Kolok, 1999; Norin & Clark, 2016; Plaut, 2001). While previous studies have shown that Ucrit is not affected by triploidy in several salmonid species (Bernier et al., 2004; Lijalad & Powell, 2009; Riseth et al., 2020; Scott et al., 2015; Small & Randall, 1989; Stillwell & Benfey, 1997), this has not been examined in S. alpinus.
In this study, we examine the effects of triploidy on the response of this polar species to exhaustive exercise through a Ucrit test with post-exercise blood sampling. Based on previous studies in other salmonids, we hypothesized that Ucrit would not differ between diploids and triploids. We sought to supplement this information with measurements of secondary stress response (blood glucose, lactate and haematocrit), and to investigate the presence, and potential causes, of ENS in S. alpinus. Such information can assist in developing species-specific farm management practices for this relatively understudied salmonid aquaculture species.
2 MATERIALS AND METHODS
2.1 Ethics statement
This research was approved by the University of New Brunswick (UNB) Animal Care Committee (Animal Use Protocol 24007) and followed all applicable welfare and experimental guidelines of the Canadian Council on Animal Care.
2.2 Fish
Diploid and triploid S. alpinus embryos were purchased at the eyed-egg stage from the Valorēs breeding program (Shippagan, NB, Canada). They were progeny of three dams (pooled eggs) and 10 sires (pooled milt) artificially spawned in April 2023, with triploidy induced in approximately half of the eggs via hydrostatic pressure treatment of 5 min at 65.5 MPa beginning 210°C-min post-fertilization and the remainder retained as diploid controls. Survival to the eyed stage (prior to shipping) was 58 ± 1% for triploids and 77 ± 1% for diploids. Fish were subsequently reared at the UNB aquatic facility (Fredericton, NB, Canada) following standard husbandry procedures (Jobling et al., 2010), with diploids and triploids reared separately in flow-through tanks supplied with dechlorinated municipal water. Mortality at UNB was not recorded but was low and with no obvious difference between ploidies.
In January 2024, all fish were moved to 314-L ploidy-specific tanks within a six-tank recirculating aquaculture system (RAS) for the months preceding experimentation. Water quality parameters in this RAS were maintained within recommended ranges (total ammonia nitrogen and nitrite both <1.0 mg/L, nitrate 0–400 mg/L, alkalinity 50–300 mg/L, hardness >100 mg/L and pH 6.5–8.5; Timmons et al., 2018) with the exception of low alkalinity (43 ppm) on a single day that was immediately corrected by increasing makeup water to the RAS. Temperature and dissolved oxygen averaged 10.3°C ± 1.0°C and 93% ± 5% of air saturation, respectively, for the entire time in the RAS, including through to the end of the experimental period. Stocking density was approximately 109 kg m−3 at the time of experimentation, with individuals weighing approximately 160 g on average (Table 1). All fish used for this experiment came from a single diploid and single triploid tank. Twenty days prior to experimentation, the seasonally adjusted photoperiod in the room was increased to 13:11 h (light:dark) from 12:12 to mimic the seasonal trends of early summer for the sake of broodstock maintained within a separate RAS, and this 13:11 regime was maintained throughout the experimental period.
TABLE 1. Critical swimming velocity (Ucrit) and parameters measured immediately after fish reached Ucrit in juvenile diploid (2n) and triploid (3n) Salvelinus alpinus (mean ± standard deviation, n = 12 per ploidy).
2.3 临界游泳速度协议
试验于 2024 年 6 月 5 日开始,连续持续 24 天。每天完成一次试验,在三倍体和二倍体鱼之间交替进行,每个倍性总共 12 条鱼。水下 30 升游泳隧道 (Loligo Systems) 用于确定 U暴击,速度通过流量计校准(Streamflo 430;尼克松流量计)。潜水泵与一个开放端口串联使用,这两个端口都位于电机驱动螺旋桨的正前方,以连续将淡水从外罐交换到游泳隧道中(10.7 L min−1).来自相邻头罐的恒定充气水通过外罐泵送,以减轻游泳器材产生的任何热量并确保稳定的氧气水平。溶解氧(Pro20;YSI)和温度(可追溯 4015;Cole-Parmer)在外罐中测量。在 24 天的测试期间,游泳隧道装置的平均温度和溶解氧水平分别为 14.9°C ± 0.7°C 和空气饱和度的 98% ± 2%。
将带有反射表面的泡沫板夹在外池的外表面,以防止视觉刺激影响游泳行为。使用游泳室内的自粘尺来估计每条鱼的叉长(LE),从而计算游泳速度(LEs−1)以尽量减少实验前的处理压力。这个 LE高估了真正的前叉长度(LF;游泳试验完成后测量)平均下降 3.9%。通过游泳池的透明盖子从上方观察鱼,特别注意不要惊动鱼。
游泳试验每天在 0900 至 0920 之间开始,从其储备池中随意挑选当天的鱼,并放置在游泳隧道的游泳室(尺寸为 55 × 14 × 14 厘米)中。该种群中的剩余鱼在取出测试鱼后立即喂食,从而确保在选择鱼进行实验之前,每个种群中始终保留饲料 24 小时。一旦被放置在游泳隧道中,鱼以 1.0 升的速度给予 1 小时的适应期Es−1然后速度提高了1.0 LEs−1每 10 分钟一次,遵循 Scott 等人(2015 年)的斜坡方案。这种情况一直持续到鱼筋疲力尽,这被定义为无法逆流游泳并被迫在游泳池末端的金属格栅上至少 3 秒。与 Scott 等人(2015 年)不同,我们没有将鱼作为 U 的一部分第二次游泳暴击协议; 相反,记录时间和游泳速度,关闭电机,立即将鱼从游泳隧道中取出并用苯佐卡因(4-氨基苯甲酸乙酯,0.05 g L)麻醉−1)在充气的 3 L 浴中。电机关闭后,鱼在腔室地板上保持一动不动,表现出疲惫的迹象,例如快速鳃盖运动,并且对处理反应最小。24 条鱼中的两条(均为三倍体)在 U 处失去平衡暴击;这些鱼在移除和随后的麻醉之前被允许在没有流动的游泳隧道中恢复。
2.3 Critical swimming velocity protocol
Trials began on 5 June 2024 and continued for 24 consecutive days. One trial was completed per day, alternating between a triploid and diploid fish, for a total of 12 fish per ploidy. A submerged 30-L swim tunnel (Loligo Systems) was used to determine Ucrit, with velocity calibrated via a flow meter (Streamflo 430; Nixon Flowmeters). A submersible pump was used in tandem with an open port, both located immediately before the motor driven propeller, to continuously exchange fresh water from the outer tank into the swim tunnel (10.7 L min−1). A constant flow of aerated water from an adjacent head tank was pumped through the outer tank to mitigate any heat generated by the swimming apparatus and to ensure stable oxygen levels. Dissolved oxygen (Pro20; YSI) and temperature (Traceable 4015; Cole-Parmer) were measured in the outer tank. Average temperature and dissolved oxygen levels in the swim tunnel setup over the 24-day test period were 14.9°C ± 0.7°C and 98% ± 2% of air saturation, respectively.
A foam board with a reflective surface was clamped to the exterior face of the outer tank to prevent visual stimuli from influencing swimming behaviour. A self-adhesive ruler within the swimming chamber was used to estimate each fish's fork length (LE) and thereby calculate swimming velocity (LEs−1) to minimize handling stress before beginning the experiment. This LE overestimated the true fork length (LF; measured after the swimming trial was completed) by an average of 3.9%. Fish were observed from above through the transparent lid of the swimming chamber, with special care taken not to startle the fish.
Swimming trials began between 0900 and 0920 every day with the fish of the day haphazardly chosen from its stock tank and placed in the swimming chamber (dimensions 55 × 14 × 14 cm) of the swim tunnel. The remaining fish in that stock tank were fed immediately after removing the test fish, thereby ensuring that feed was always withheld from each stock tank for 24 h prior to selecting a fish for experimentation. Once placed in the swim tunnel, the fish was given a 1-h habituation period at a velocity of 1.0 LEs−1 and then the velocity was increased by 1.0 LEs−1 every 10 min, following the ramping protocol of Scott et al. (2015). This continued until the fish was exhausted, which was defined as inability to swim against the current and being forced onto the metal grate at the end of the swimming chamber for at least 3 s. Unlike Scott et al. (2015), we did not swim the fish a second time as part of the Ucrit protocol; rather, the time and swimming velocity were recorded, the motor was switched off, and the fish was immediately removed from the swim tunnel and anaesthetized with benzocaine (ethyl-4-aminobenzoate, 0.05 g L−1) in an aerated 3-L bath. Fish remained motionless on the chamber floor after the motor was turned off, displaying signs of exhaustion such as rapid opercular movements and reacting minimally to handling. Two of the 24 fish (both triploids) lost equilibrium at Ucrit; these fish were allowed to recover in the swim tunnel with no flow before removal and subsequent anaesthesia.

